Page 8 - hypertension_newsletter4_Final
P. 8
REFLECTIONS
Hypertension
Hypertension Global Newsletter #4 2023
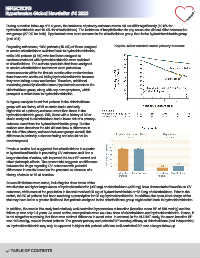
During a median follow-up of 2.4 years, the incidence of primary-outcome events did not differ significantly (10.0% for
hydrochlorothiazide and 10.4% for chlorthalidone). The incidence of hospitalization for any cause also did not differ between the Hypertension
two groups (27.0% for both). Hypokalemia was more common in the chlorthalidone group than in the hydrochlorothiazide group
(p<0.001).
Regarding adherence, 1039 patients (15.4%) of those assigned Kaplan–Meier survival curve primary outcome
to receive chlorthalidone switched back to hydrochlorothiazide,
while 260 patients (3.8%) who had been assigned to
continue treatment with hydrochlorothiazide were switched
to chlorthalidone. The authors speculate that those assigned
to receive chlorthalidone underwent more potassium
measurements within the first six months after randomization
than those who continued taking hydrochlorothiazide because
they were taking a new medication. Therefore, additional
monitoring probably identified more hypokalemic events in the
chlorthalidone group, along with any new symptoms, which
prompted a switch back to hydrochlorothiazide.
Subgroup analysis found that patients in the chlorthalidone
group with no history of MI or stroke had a modestly
higher risk of a primary-outcome event than those in the
hydrochlorothiazide group. Still, those with a history of MI or
stroke assigned to chlorthalidone had a lower risk of a primary-
outcome event than the hydrochlorothiazide group. But the
authors note that since the trial did not show a difference in
the risk of the primary outcome between groups overall, this
difference is probably a chance finding and should not be
overinterpreted.
Previous studies had suggested that chlorthalidone is superior
to hydrochlorothiazide in preventing CV outcomes as it has a
longer duration of action, with improved 24-hour BP control and
other pleiotropic effects. The present trial suggests no difference
between the drugs regarding CV outcomes with potential
differences in results based on the presence or absence of a
history of stroke or MI at baseline.
Several limitations were noted, including the dose levels of the
two diuretics as higher target doses of hydrochlorothiazide (≥50 mg) or chlorthalidone (≥25 mg) have demonstrated benefits on CV
outcomes, while most of the population in the trial received 25 mg of hydrochlorothiazide or 12.5 mg of chlorthalidone. Prior to the
switch, 94.5% of patients had been receiving a prescription for 25 mg hydrochlorothiazide. In addition, the open-label design of the
trial may have led to a greater likelihood that patients assigned to the chlorthalidone group might switch back to hydrochlorothiazide.
In addition, the men in this study had relatively well-controlled hypertension at baseline (baseline mean BP of 139 mmHg) and the
follow-up was only 2.4 years. As noted earlier, most patients were on a low dose of chlorthalidone and hydrochlorothiazide. Hence, it
is not altogether surprising that there was minimal difference in event rates. In contrast, in the ALLHAT study, the mean baseline BP
was 145 mmHg, even in treated patients. The greater potency and potential BP lowering efficacy of chlorthalidone (and indapamide)
vs. hydrochlorothiazide may only be apparent in higher risk patients with less well-controlled BP over a longer follow-up.
TABLE OF CONTENTS